Last time, we revealed how biomass makes its own water during chemical dehydration. But that’s not the end of the story for that steam. It becomes a key player in in-reactor steam reforming reactions, shaping your final product mix and process efficiency.
So, what is steam reforming and why is it so critical?
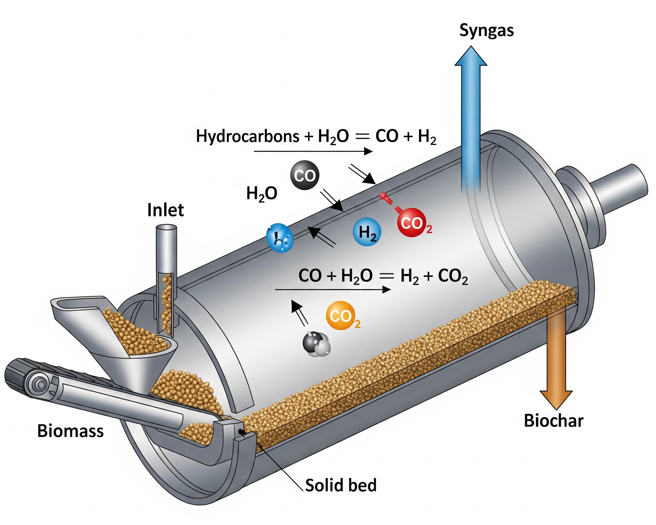
Simply put, steam reforming is the high-temperature reaction of steam with volatile organic compounds—specifically tars and light hydrocarbons—to produce a cleaner syngas. It’s a powerful tool for process control, but its use is a delicate balancing act. Here’s why:
– Tar Reduction: This is its most significant role. Heavy tars, which can clog equipment and foul catalysts, are cracked into smaller molecules by the steam.
– Syngas Quality: Steam reforming dramatically impacts syngas composition. It promotes the water-gas shift reaction, which increases the valuable hydrogen (H2) content. However, this often comes at the expense of carbon monoxide (CO) and methane (CH4), which can reduce the overall heating value of your syngas.
This is where moisture content is key. The amount of water in your feedstock is not just a liability; it is the genesis of this chemical story.
– Too much moisture and the excess energy needed for evaporation and the endothermic reforming reactions can make your process uneconomical. This high moisture can also lead to the formation of undesirable, heavy tars that are more prone to deposition.
– Too little moisture, and you may have insufficient steam for effective reforming, leading to higher overall tar production, reactor clogging, and a lower-quality syngas.
This is why there is a crucial optimal moisture content window. Within this window, the moisture provides just enough in-situ steam to assist in tar cracking and produce a balanced, high-value syngas without crippling your process’s energy balance.
The effectiveness of this process is also dictated by several critical factors:
– Temperature Dependence: Reforming reactions are highly endothermic and require high temperatures to proceed effectively.
– Catalysts: The presence of catalysts can dramatically lower the required temperature and improve reaction efficiency.
– Gas Residence Time: The average time a gas molecule spends in the reactor’s hot zone is crucial for allowing these reactions to occur.
Ultimately, this is a story of economics and operational utilisation as much as it is of chemistry. Whether you’re a small operator looking to reduce downtime and improve efficiency, or a large organisation aiming to open new high-value markets by turning hydrogen-rich syngas into advanced liquid fuels via Fischer-Tropsch synthesis, optimising these chemical pathways allows us to unlock the full economic potential of biomass.
Have you found certain catalysts or operating parameters to be particularly effective in optimizing steam reforming? Share your insights and experiences below!
The Pyrolysis Steam Story: From Dehydration to Reforming