Beyond just “drying,” what happens when biomass creates its own water during pyrolysis?
Last week, we discussed the nuances of moisture content and its impact on heat transfer. This week, let’s peel back another layer and dive into chemical dehydration, a fascinating but often overlooked transformation that profoundly shapes your bio-oil, biochar, and syngas.
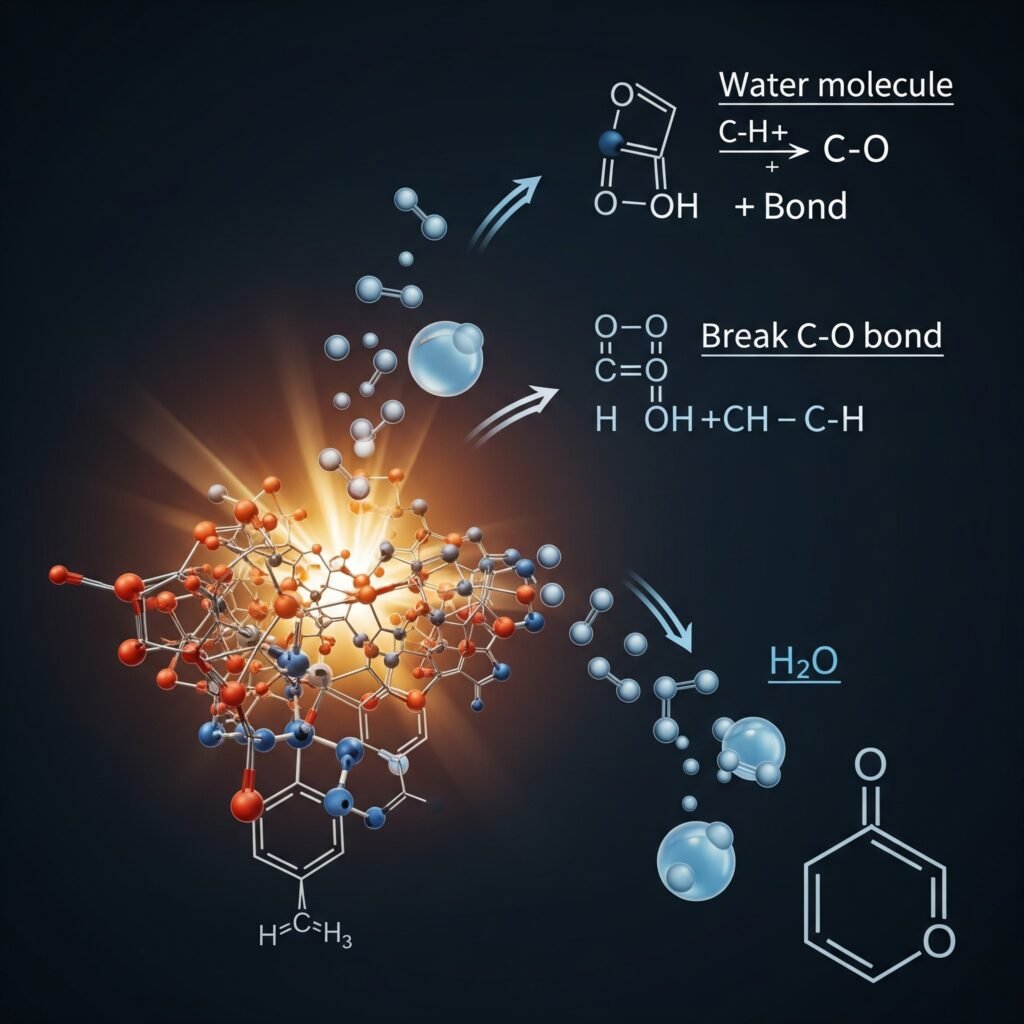
Imagine your biomass feedstock as a complex molecular puzzle. During physical drying, we’re simply removing the water molecules sitting on its surface or trapped in its pores. But as we ramp up the temperature, say above 150°C, the biomass itself starts to reconfigure. This is where chemical dehydration comes in: it’s the process where water molecules are formed and then released from within the biomass’s molecular structure. Essentially, hydrogen and oxygen atoms from neighboring hydroxyl (-OH) groups (alcohol-like bits abundant in plant matter) combine, chemically bonding to create a molecule of water, which then escapes as vapor.
Why is this subtle chemical process so critical for your thermal conversion operations?
– Tar Formation & Quality: Chemical dehydration significantly influences the pathways that lead to different tar compounds. This can impact everything from your bio-oil’s viscosity to its energy density.
– Reaction Kinetics & Product Distribution: The removal of these hydroxyl groups, and the subsequent “holes” left in the biomass structure, can kickstart or accelerate other critical pyrolysis reactions. This directly affects your char yield, gas composition, and the overall efficiency of your process.
– Energy Balance Re-evaluation: While not latent heat, these reactions can be endothermic, adding another layer of complexity to your process’s energy profile.
Understanding this intricate chemical dimension of dehydration is crucial for truly optimizing biomass pyrolysis. It allows us to move beyond simply managing “wetness” and delve into controlling the intrinsic chemical pathways that dictate our product yields and qualities.
This chemical transformation isn’t just theory; it has tangible impacts on everything from the types of bio-oil components produced to the stability of your char. It’s a key part of the biomass pyrolysis story we’re continuing to unravel.
Next time, we’ll explore how these newly formed water molecules, along with any residual moisture, play a role in steam reforming reactions within your reactor – another critical aspect often overlooked!
What analytical techniques or operational strategies have you found most effective for understanding or managing chemical dehydration in your biomass thermal processes? Share your insights and experiences below!
Beyond Drying: The Hidden Story of Chemical Dehydration in Biomass Pyrolysis